Organ Remodeling: Chemical Exposure in Non-Target Organisms
Taking advantage of the existing strengths in our department, a project examining the effects of herbicide-exposure (atrazine) during amphibian organ formation was initiated. Why study atrazine exposure on non-target organisms? Originally intended to block photosynthesis in plants, atrazine is currently one of the most widely used herbicides in the United States. Thus, when released for use over 50 years ago, it was believed that animals would be immune to the deleterious effects observed in plants. However, the observation of atrazine-induced defects in several non-target organisms indicated that this hypothesis was false. Since amphibians are highly sensitive to pollutants in the environment, they provide one of the first indicators of damage to an ecosystem. The exact effects of atrazine on amphibian populations are currently under serious debate. Some researchers have demonstrated that exposure of tadpoles to very low doses of atrazine converts male frogs into hermaphrodites (Hayes, 2004) and atrazine is a dangerous endocrine-disrupting chemical (Carr et al., 2003; Hayes at al., 2002). Still, other research teams do not observe this hermaphrodite-phenotype, or any phenotype, in the atrazine-exposed adults (Solomon et al., 1996). Further studies are needed to determine what role, if any, atrazine is playing in the decline in amphibian populations observed worldwide.
It is not surprising that anthropogenic pollution in the environment can lead to undesirable consequences for non-target organisms that are less overt than direct toxicity. Unlike many of the previous studies that measured late endpoints (reproductive development, metamorphosis, or survival), members of my lab are focusing on earlier, and perhaps more subtle, anomalies by examining organ patterning and morphogenesis in premetamorphic tadpoles. The contradictory results of earlier published research, in combination with the relatively late and severe endpoints typically assayed in previous studies, have left many issues unaddressed. Accordingly, to carefully assess the teratogenicity of atrazine in non-target organisms, we utilized the existing strengths and expertise (organ development) in our lab and initiated a systematic examination of the consequences of atrazine exposure during organ development.
Taking an interdisciplinary approach (populations through molecules) to this controversial issue allows us to begin to understand the possible impact this class of chemicals has on biological systems. It would be of great value to identify the molecular mechanisms activated following atrazine exposure that result in the phenotypes observed (for example see Fig. 4). Because my lab already has the ability to examine normal development of embryonic organs (both at a morphological and molecular level), we can use this expertise to characterize the underlying mechanisms resulting in organ anomalies caused by this environmental toxin. Results obtained from these studies will be of interest to many different scientific communities interested in xenobiotic substances as well as organogenesis.
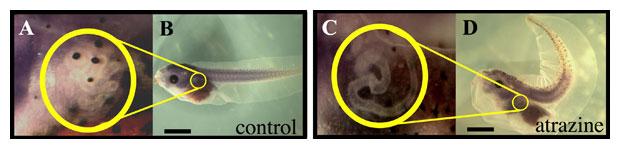
Figure 4: Atrazine induced phenotypes - Axis and renal (pronephric) malformations. (A-B) Lateral view of a feeding-stage (untreated) control tadpole. Circled area in (B) indicates location of pronephric tubules shown under higher magnification in (A). Note the normally coiled pronephric tubules located in the yellow circle. (C-D) Lateral view of atrazine-treated, feeding-stage tadpole showing a curved anterior-posterior body axis in panel (D) and diffuse, disorganized pronephric tubules shown at higher magnification in (C) when compared to the tight circle of pronephric tubules in (A).
A few examples of manuscripts from our lab describing this type of work
(*denotes undergraduate research assistant):
- Lenkowski, J.R., Sanchez-Bravo*, G. and McLaughlin, K.A. (2010) Low concentrations of atrazine, glyphosate, 2,4-dichlorophenoxyacetic acid (2,4-D), and triadimefon exposures have diverse effects on Xenopus laevis organ morphogenesis. Journal of Environmental Sciences 23(1).
- Lenkowski, J.R. and McLaughlin, K.A. (2010) Acute atrazine exposure disrupts matrix metalloproteinases and retinoid signaling during organ morphogenesis in Xenopus laevis. Journal of Applied Toxicology. 30(6) 582-589.
- Lenkowski, J., Reed, J.M., Deininger, L.*, and McLaughlin, K.A. (2008) Perturbation of Organogenesis by the Herbicide Atrazine in the Amphibian Xenopus laevis. Environmental Health Perspectives. 116:223-230.
If you want to learn more about this project, please contact Kelly McLaughlin.