Evolution of Reproductive Isolation
During speciation, the transition from weak to strong reproductive isolation generally requires the evolution of multiple barriers to gene exchange between populations. ‘Coupling’ has emerged as a concept to describe the processes and mechanisms causing distinct barriers to accumulate and operate together, with three main forms being recognized: selection, population processes, and genetic architecture (Fig. 1). Although coupling dynamics have been explored for many decades in the theoretical literature, we need more empirical studies that establish the processes responsible for the accumulation of barriers to gene exchange in natural systems. Do distinct barriers usually accumulate as an adaptive response or as a by-product of other processes? How important is chromosomal linkage, or traits contributing to multiple barriers? Does coupling tend to involve a consolidation of local barriers scattered across the distribution range or an evolutionary response to complex environments at single locations? Since reductions of gene flow between populations depend in part on both the origin of barriers to gene exchange and the ways in which they are coupled together, answers to these questions are key to an understanding of speciation.
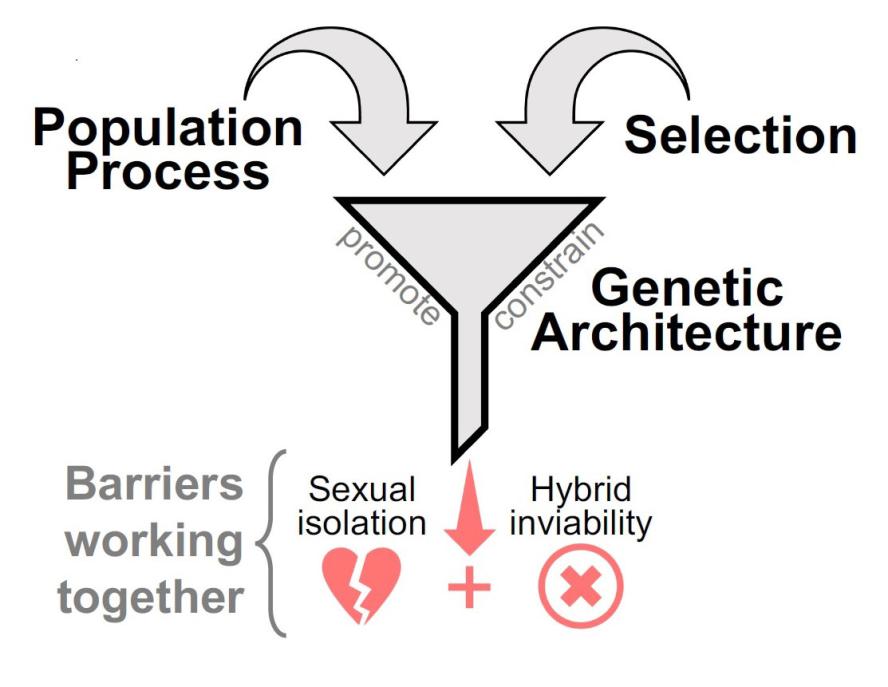
Fig. 1. We are interested in examining all three central coupling processes to uncover the mechanisms underlying the tendency of different barriers to operate together. To illustrate, if two barriers (sexual isolation, hybrid inviability) define the same population pairs, a stronger overall barrier to gene exchange can be established compared to population pairs that differ in one barrier effect only.
Selected Publications
- Dopman, E.B., Shaw, K.L., Servedio, M.R., Butlin, R.K. and Smadja, C.M., 2024. Coupling of barriers to gene exchange: Causes and consequences. Cold Spring Harbor Perspectives in Biology, p.a041432.
- Firneno, T.J., Semenov, G., Dopman, E.B., Taylor, S.A., Larson, E.L. and Gompert, Z., 2023. Quantitative Analyses of Coupling in Hybrid Zones. Cold Spring Harbor Perspectives in Biology, 15(12), p.a041434.
- Kunerth, H.D., Bogdanowicz, S.M., Searle, J.B., Harrison, R.G., Coates, B.S., Kozak, G.M. and Dopman, E.B., 2022. Consequences of coupled barriers to gene flow for the build-up of genomic differentiation. Evolution, 76(5), pp.985-1002.
- Coates, B.S., Kozak, G.M., Seok Kim, K., Sun, J., Wang, Y., Fleischer, S.J., Dopman, E.B. and Sappington, T.W., 2019. Influence of host plant, geography and pheromone strain on genomic differentiation in sympatric populations of Ostrinia nubilalis. Molecular Ecology, 28(19), pp.4439-4452.
- Dopman, E.B., Robbins, P.S. and Seaman, A., 2010. Components of reproductive isolation between North American pheromone strains of the European corn borer. Evolution, 64(4), pp.881-902.
- Dopman, E.B., Pérez, L., Bogdanowicz, S.M. and Harrison, R.G., 2005. Consequences of reproductive barriers for genealogical discordance in the European corn borer. Proceedings of the National Academy of Sciences, 102(41), pp.14706-14711.